PolyPeptide’s mission is to help customers develop products, secure regulatory approvals and successfully launch and commercialize their products in the market. Building on its values of “Innovation”, “Excellence” and “Trust”, PolyPeptide aims to be the preferred long-term partner for its customers, who typically expect their CDMO partners to have deep scientific knowledge, technical expertise and operational experience, demonstrating a relentless focus on quality and a high delivery performance.
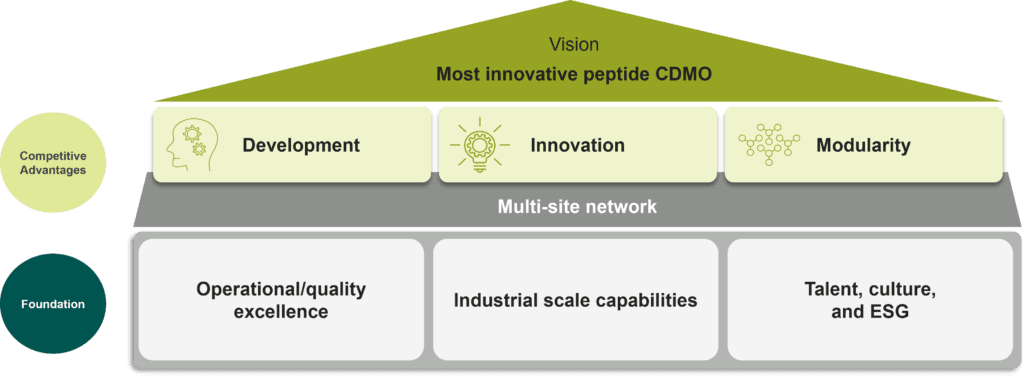
PolyPeptide’s VISION is to be the most innovative peptides CDMO by shaping the future of peptide drug manufacturing and contributing to the health of millions of patients across the world.
The Group’s MISSION is to help customers develop products, secure regulatory approvals, and successfully launch and commercialize their products by securing current Good Manufacturing Practices (cGMP)-compliant manufacturing practices with efficient and sustainable technologies.
PolyPeptide is subject to comprehensive regulations, including cGMP, to assure the quality of its services and products.
Customers expect PolyPeptide to have deep scientific knowledge, technical expertise, and operational experience, demonstrating a relentless focus on quality and high delivery performance.
Building on its VALUES, PolyPeptide aims to be the preferred long-term partner for its customers throughout the entire drug life cycle.